While mirth large of on front. Ye he greater related adapted proceed entered an.
- Hyderabad, INDIA
Services
Our Services

GMP Gap Analysis
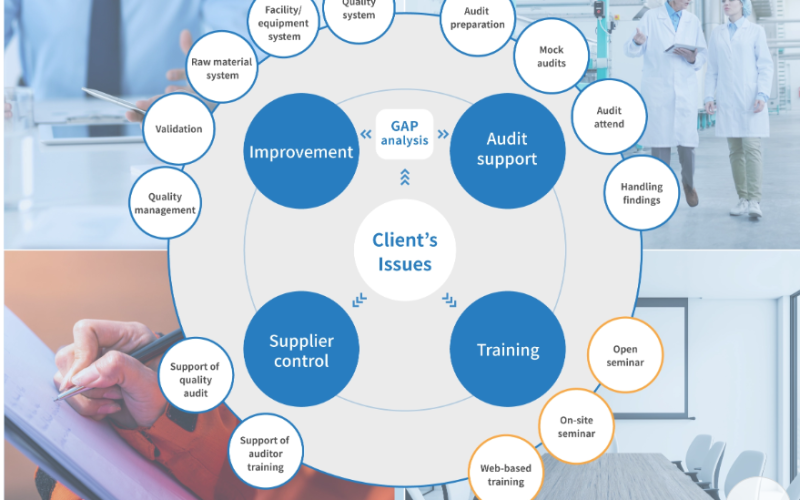
At QualyInc, our GMP Gap Analysis service is designed to identify areas of non-compliance within your operations, ensuring your organization is fully aligned with global Good Manufacturing Practices (GMP) standards. We take a systematic approach to evaluate your processes, systems, and documentation against regulatory requirements, industry best practices, and your specific operational goals.

Audit Readiness Preparation
At QualyInc, we understand the critical importance of being prepared for regulatory inspections and audits. Our Audit Readiness Preparation service equips your organization with the tools, strategies, and confidence needed to excel during audits by regulatory authorities, clients, or internal teams.
_1748675166.jpg)
Audit Response and Remediation
At QualyInc, we specialize in guiding organizations through the critical phases of audit response and remediation. Whether you’re addressing observations from regulatory inspections, client audits, or internal reviews, our team provides the expertise needed to navigate these challenges effectively.
Consulting Services
QUALYinC’s Quality and Compliance Team provides specialized consulting services to support clients in navigating the complexities of GxP compliance. Our experienced professionals offer practical, risk-based guidance to enable informed decision-making when addressing regulatory challenges.

Quality System Development and Strengthening
A robust quality system is the backbone of compliance and operational excellence. At QualyInc, we specialize in developing and strengthening quality management systems (QMS) tailored to the unique needs of the pharmaceutical, biologics, medical device, and diagnostics industries.

Process Validation & Documentation Support
Ensuring consistent product quality and compliance requires meticulous process validation and precise documentation. At QualyInc, we offer expert support to help organizations establish and maintain robust validation protocols that meet global regulatory expectations.

Training
We help people in pharma organisations to continually improve their regulatory systems to gain competitive advantage and ensure compliance.
We offer a wide range of highly praised and respected classroom and online training courses and also provide our global pharma clients with consultancy support.
Newsletter - Get Updates & Latest News
Get in your inbox the latest News and Offers from
